Conditioned Media from Human
Umbilical Cord-derived Stem CellBRUWS
Confirm
This page is intended to provide information to those involved in medical institutions,
cosmetics or health food industries, and those engaged in related business.
It is not intended to provide information to general consumers.
Are you involved in medical institutions, cosmetics,
health food industries,or any related business?
- NO
- YES
Conditioned Media from Human
Umbilical Cord-derived Stem CellBRUWS



What is “Conditioned Media from Human Umbilical Cord-derived Stem Cell”?
1. Human Stem Cell-derived Conditioned Media
Human Stem Cell-derived Conditioned Media is supernatant liquid extracted
in the process of culturing human stem cells.
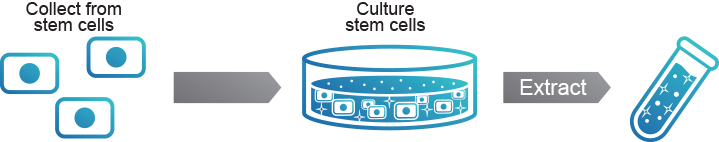
- POINT1
- Cells are not included in conditioned media.
- POINT2
- Cytokine secreted from stem cells is included abundantly.
2. Cytokine
It is a type of functional protein secreted by stem cells and exists in large numbers.
Having a particularly strong effect of encouraging cell growth are called growth factors.

Paracrine effect:
Cytokines are having various functions by transmitting information between cells to encourage proliferation and differentiation of surrounding radio waves, suppression of inflammation, and improvement of mimicry.
3. Cytokine Orchestra
The idea that many cytokines with different properties interact with each other.
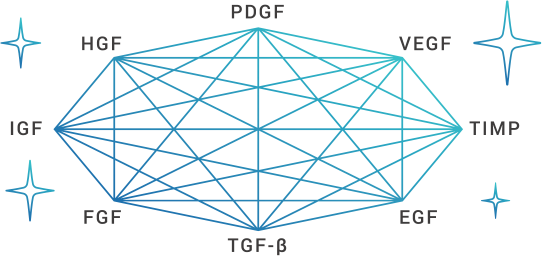
- POINT1
- Each cytokine has different characteristics and concentration.
- POINT2
- There is a meaning in the totality of a group of cytokines.


Comparison of Cytokines
Representative cytokines are as follows.
Research Reagent Product:
Conditioned Media from Human Adipose-derived Stem Cell
(Product Code: CPJ1)
PDFF-BB: Platelet-Derived Growth Factor-BB
- Work on angiogenesis
- Advance to regenerate aged skin
(Improving wrinkles and flabbiness.)
HGF: Hepatocyte growth factor
- Work on wound healing
Angiopoietin: Angiogenetic factor
- Work on angiogenesis
Collagen
- Advance to regenerate aged skin
(Improving wrinkles and flabbiness.)
Research Reagent Product:
Conditioned Media from Human Umbilical Cord-derived Stem Cell
(Product Code: CPJ2)
IGF1: Insulin-like growth
- Advance to osteogenesis
- Advance to regenerate aged skin
(Improving wrinkles and flabbiness.)
FGF2: Fibroblast growth factor
- Work on angiogenesis and wound healing
TIMP2: Tissue inhibitor of metalloproteinase-2
- Work on angiogenesis and wound healing
EGF: Epidermal growth factor
- Advance to wound healing
- Advance to regenerate aged skin
(Improving wrinkles and flabbiness.)
PDFF-AA: Platelet-Derived Growth Factor-AA
- Work on wound healing
IL10: Interleukin-10
- Have an inhibitory effect on inflammatory response


Striving for Safety
We provide conditioned media which cleared in the strict three-step inspection standards.
1. Conducting inspections to Japanese donors: Donor Screening
HBV, HCV, HTLV, PVB19, Syphilis > ALL NEGATIVE
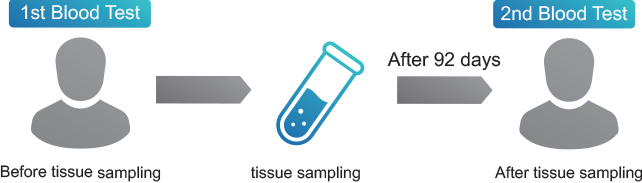
- We conduct two blood tests to donors to make sure there are no infectious diseases.
- These inspections are available since we are identifying donors.
- Window period: In accordance with the Ministry of Health, Labor and Welfare of Japan
- Examination item of infectious diseases
2. Conducting inspections to culturing cells
(1) Virus inspection
HBV, HCV, HTLV, PVB19, CMV, EB, WNV > ALL NEGATIVE
- We confirm that the cell is not infected by a virus in the process of culturing.
- Virus inspection items:
In accordance with the Ministry of Health, Labor and Welfare of Japan - In addition to the usual virus test items, the coronavirus test (PCR method) has also been conducted on the stem cells used in the production process of the culture supernatant to confirm that they are negative.
Since the coronavirus test (PCR method) is temporarily introduced, it may end without notice.
(2) Mycoplasma test
- We confirm that cells are not contaminated by mycoplasma in the process of culturing.
3. Conducting inspections to extracted conditioned media
(1) Sterility test
- We confirm that the conditioned media are not including fungus and bacteria.
(2) Endotoxin test
- We confirm the conditioned media’s endotoxin concentration is below 0.5 EU/ml.
How to order
Please send us an email to info2@birthbank.co.jp with the following items.
1. Product code / 2. Order quantity / 3. Preferred delivery date and time /
4. Delivery address and PIC‘s phone number.
Our PIC will contact you within a day.
| Adipose-derived (Product Code: CPJ1) |
Umbilical Cord-derived (Product Code: CPJ2) |
|
|---|---|---|
| Price /ml | Please contact. | Please contact. |
| Order unit | 10 tubes (1ml x 10 tubes) | |
| Payment | Bank transfer *Please kindly bear the bank transfer fee. |
|
| Delivery | On Mondays and Wednesdays *ETA will be differed depends on the delivery area. |
|
| Order deadline | Please order 7 days before our shipment date. | |
| Shipping company | By Yamato Transport or Sagawa Express. (We don’t export outside of Japan.) | |
| Shipping fee | A flat rate: free in Japan. (Chilled and frozen parcel delivery service) | |
| Storing method | Lower than -40℃(ideally lower than -70℃.) Please note that the expiration date will become shorter if stored in a household-use fridge at around -18°C. |
|
| Expiration date | Stored at lower than -40℃(ideally lower than -70℃): within 6 months after the delivery Stored in a household-use fridge at around -18°C: within 2 weeks after the delivery |
|
FAQ
- Q.Is any notification required when using at medical institutions?
- A.It is unnecessary since there is no specific law regarding conditioned media in Japan at this moment. Conditioned media are neither a drug nor a product such as regenerative medicine. We offer this as a research reagent product.
- Q.Is refreezing available?
- A.We don’t recommend to do so.
The numbers of cytokines will decrease by repeating dissolving and refreezing.
- Q.Is it impossible to donate blood if you even once administer the conditioned media?
- A.Yes, by regulation of Japan Red Cross Society.
Should you have any questions,
please kindly let us know.
It is not intended to prove the efficacy of products.

